Biogeochemical Cycles
Key Takeaways Biogeochemical cycles are crucial natural processes, which continuously recycle vital nutrients in different forms and locations, linking all living organisms with the physical environment, and sustaining life on Earth. A deeper understanding of these biogeochemical cycles will allow us to understand their importance in maintaining nutrient availability and ecosystem health. The carbon cycle is fundamental to regulating climate and includes important exchanges between land, ocean, and atmosphere. Human activities like burning fossil fuels have thrown this cycle completely out of whack. All of this makes it imperative that we begin applying strategies to reduce these impacts. The nitrogen cycle helps maintain the health of ecosystems that provide plant growth and soil fertility through processes such as fixation and nitrification. However, human interventions, especially in agriculture, can cause nitrogen pollution, making the case for sustainable agricultural pra
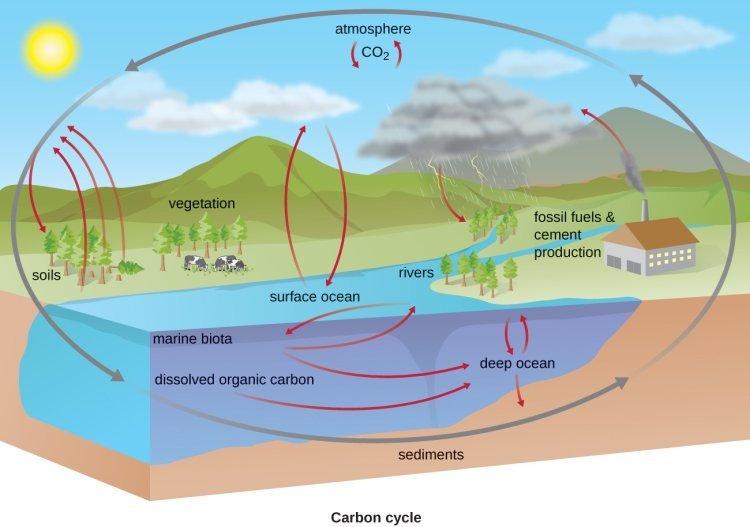
Understanding biogeochemical cycles helps us understand how major processes like the carbon cycle and nitrogen cycle are important to our planet and society.
These biogeochemical cycles transport critical compounds like carbon and nitrogen through environments, fueling all living things.
Human activities break these natural flows, resulting in an accumulation of greenhouse gases and adverse effects on our ecosystem.
By better understanding these cycles, we can begin to tackle our biggest environmental challenges.
With a few basic practices, we can all do our part to lessen our footprint and help create a cleaner, more sustainable world.
Join us on our journey to explore the incredible world of nature’s cycles and discover how we can all get plugged in!
What Are Biogeochemical Cycles
Biogeochemical cycles are vital processes that recycle matter through ecosystems. They keep life going by circulating elements in different forms and through different places. These cycles constantly circulate and change the chemical forms and compounds.
They include complex and dynamic interactions between the biosphere, atmosphere, and earth’s lithosphere. On Earth, the matter is limited and conserved. This recycling aspect is especially critical since most elements are finite in nature, as they are reused and cycled to sustain life on earth.
Our Law of Conservation of Mass reminds us that the movement of matter is cyclical. It claims that matter is neither created nor destroyed, only changed. The important chemical elements in these cycles are referred to by the acronym CHNOPS.
These nutrients include carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. These elements are fundamental to all forms of life, supporting the vast array of complex ecosystems that exist on Earth.
1. Definition and Importance
Biogeochemical cycles are the important pathways that connect the biotic (living) and abiotic (non-living) elements of ecosystems. By making key nutrients accessible, they allow organisms to grow and flourish, providing a balance to our ecosystems.
Eventually, elements settle down into sinks, such as oceans and forests. They are emitted from natural sinks, like volcanic eruptions and the burning of fossil fuels. Carbon is currently sequestered in the terrestrial subsurface.
This storage accounts for nearly half of Earth’s carbon and has a profound effect on nutrient availability. These cycles tremendously enrich the available nutrients. They renew important nutrients in the earth and aquatic waters that all living things rely on to develop and reproduce.
In semi-aquatic ecosystems such as rice paddies, nitrogen-fixing cyanobacteria are essential contributors to soil fertility. Through their activity, they demonstrate the real-world relevance of these critical cycles.
2. Types of Biogeochemical Cycles
For gaseous cycles such as carbon and nitrogen, the atmosphere is a major reservoir. These cycles are also characterized by a much quicker turnover.
Sedimentary cycles, like the phosphorus cycle, are linked with the Earth’s crust. These are longer-term processes, such as weathering of rocks, which may take millions of years.
These cycles can further be categorized by their main components. The carbon cycle, for example, includes carbon being exchanged between the ocean and atmosphere, an exchange that can take hundreds of years.
Here is a simplified table summarizing the key features:
|
Cycle Type |
Primary Element |
Reservoir |
Turnover Rate |
|---|---|---|---|
|
Gaseous |
Carbon |
Atmosphere |
Centuries |
|
Gaseous |
Nitrogen |
Atmosphere |
Decades |
|
Sedimentary |
Phosphorus |
Earth's crust |
Millions of years |
3. Role in Ecosystems
Biogeochemical cycles are crucial to life on Earth, recycling vital nutrients and supporting all ecosystems. Organisms go on to produce a web of interdependence.
Plants, animals, and microorganisms all depend on each other for nutrient cycling. These cycles have a profound impact on all ecosystem productivity and health, determining the availability of essential nutrients and energy flow.
When human activities release excess carbon or nitrogen into our environment, these cycles get disrupted. Such disruptions cause ecological imbalances, which harm biodiversity and ecosystem services.
Nitrogen in its molecular form is very inert, but once microorganisms fix nitrogen, it becomes indispensable. This natural process increases soil fertility and encourages lush, healthy plant growth.
The Carbon Cycle
The carbon cycle is one of the most basic and important biogeochemical cycles that is directly involved in supporting life on Earth. It is about the flow and transfer of carbon between Earth’s atmosphere, oceans, land, and all the living things on and in it. This cycle keeps the balance of carbon in all of its reservoirs, which is vital for all life processes—including photosynthesis and cellular respiration.
These biological processes are critically important for the maintenance of atmospheric oxygen and energy production that sustains ecosystems across the globe. Carbon's presence in the atmosphere as carbon dioxide (CO2) significantly influences climate regulation by trapping heat and maintaining Earth's temperature within a range conducive to life.
Carbon Exchange Between Land and Ocean
Carbon exchange between terrestrial and aquatic carbon pools happens through a number of complex pathways. These processes involve the export of carbon through rivers to the ocean, sedimentation, and the absorption of atmospheric CO2 into seawater.
Photosynthesis and respiration are primary forces in this back and forth. Combined, land and marine phytoplankton plants sequester about 120 gigatons of carbon annually through photosynthesis. Though, through autotrophic respiration, about 60 gigatons of this carbon is returned back to the atmosphere.
The ocean absorbs around 2.3 gigatons of carbon yearly, accounting for 27% of carbon emissions from fossil fuel burning, thereby playing a significant role in regulating atmospheric CO2 levels.
Factors influencing carbon exchange rates include:
-
Temperature variations affecting enzyme activity in photosynthesis.
-
Ocean currents that help rapidly mix surface and deep waters.
-
Human-induced changes in land use and oceanic conditions.
Rapid Carbon Movement in Atmosphere
Because carbon cycles rapidly through processes such as photosynthesis and respiration, it is helped by the movement through food webs. This process allows plants to recycle and cycle atmospheric CO2 into organic matter, which becomes the basis for food chains.
As organisms eat plants, carbon moves up through the various trophic levels. The ocean, the world’s largest carbon sink, absorbs more CO2, lowering levels in the atmosphere. The biosphere’s gross primary productivity and ecosystem respiration are major components of the biospheric carbon cycle.
Despite these natural processes, CO2 concentration in the atmosphere has risen sharply since 1850, currently stabilizing around 1.8 parts per million, impacting climate change dynamics.
Human Impact on Carbon Cycle
Yet, human activities—most especially the burning of fossil fuels—have thrown this delicate balance into disarray. In 2018, that means anthropogenic sources added about 36.6 gigatons of carbon to the atmosphere.
Deforestation decreases the ability of the environment to store carbon, as trees—important long-term carbon sinks—are removed, releasing the carbon stored in their biomass. This leads to greater greenhouse gas emissions, worsening global warming.
Strategies to prevent these effects are reforestation, transitioning to renewable energy sources, and developing more efficient carbon capture technologies.
The Nitrogen Cycle
The nitrogen cycle is an essential ecosystem process, which recycles nitrogen through the environment and is vital to all forms of life. As one of the key macronutrients, nitrogen is critical for all living organisms. It’s a critical part of amino acids, proteins, and nucleic acids—the basic building blocks of all living organisms.
Atmospheric nitrogen (N2) is quite inert and thus unavailable to most organisms. This is where the nitrogen cycle comes in to make this seemingly useless nitrogen useful. Nitrogen is an essential and often limiting nutrient for plant health. They also take up ammonium (NH4+) and nitrate (NO3-) from the soil.
These forms are important for plant growth. They deliver the key nutrients that feed the entire food web.
Nitrogen-fixing bacteria play a key role in the nitrogen cycle. They do this by literally pulling down atmospheric nitrogen and converting it into ammonia in a process called nitrogen fixation. That can happen either through free-living bacteria or, as is the case in the roots of legumes, through symbiotic relationships.
Interestingly enough, lightning strikes are another major driver of nitrogen fixation, producing an estimated 5 to 10 billion kilograms of fixed nitrogen each year. Bacteria, called diazotrophs, are responsible for converting most of this. Bacterial processes are also key to soil fertility. They replenish the nitrogen that plants take from the soil, making sustainable agriculture possible.
Nitrogen Conversion Processes
The nitrogen cycle is made up of many important processes, such as fixation, nitrification and denitrification. As discussed above, fixation is the process of converting atmospheric nitrogen into a form that organisms can use.
Nitrification comes next, with ammonia being oxidized first to nitrite (NO2-) and then to nitrate (NO3-) by specialized nitrifying bacteria. Denitrification finishes out the cycle by turning these nitrates back into nitrogen gas, thereby returning it to the atmosphere.
These processes also contribute to soil fertility by preventing the accumulation of excess nitrogenous compounds in the soil. Plants absorb these nitrogen compounds through their root systems, taking them up into organic molecules.
Main Steps in Nitrogen Conversion:
-
Atmospheric nitrogen fixation by bacteria or lightning.
-
Conversion of ammonia to nitrite and nitrate through nitrification.
-
Assimilation of nitrates by plants.
-
Reduction of nitrates to nitrogen gas via denitrification.
Human Influence on Nitrogen Cycle
Human activities, most particularly agriculture, have dramatically increased reactive nitrogen in ecosystems. Synthetic fertilizers have greatly increased the amount of reactive nitrogen (Nr) available.
This change has upset natural nitrogen cycles and led to widespread pollution. Increased nitrogen levels now overtake our water bodies. This shift is damaging water quality and releasing nitrogen oxides, which contribute to poor air quality.
Fertilizers, even as they help farmers grow food for a growing population, throw a wrench into the intricate cycle of nitrogen. Ways to prevent human interference include transitioning to more sustainable agricultural practices, such as using controlled-release fertilizers and improving nitrogen-use efficiency in crops.
Eutrophication and Its Effects
Eutrophication is a harmful process that is largely driven by the runoff of excess nutrients—especially nitrogen—from land to water. This nutrient overload feeds harmful algal blooms that can grow at alarming rates.
This excess nitrogen fuels algal blooms which consume available oxygen in the water, creating dead zones where aquatic life cannot live. The economic impacts of eutrophication are huge, impacting fisheries, tourism, and drinking water quality management.
Restoring that balance means making smart investments to reduce nutrient runoff by promoting more sustainable agricultural practices and improving waste management.
The Phosphorus Cycle
Perhaps the most important factor is that the phosphorus cycle is responsible for keeping biological systems in homeostasis. Unlike other biogeochemical cycles, phosphorus is different in that it is mainly sedimentary. Unlike nitrogen, phosphorus has no atmospheric component.
Rather, it is naturally sequestered in the Earth’s lithosphere, most notably in oceanic and freshwater sediments. The cycle is the movement of phosphorus through the ecosystem, aiding in the transfer of energy in the form of ATP through food chains. Phosphorus is an essential component of DNA, RNA, and ATP. This makes it critical for the growth and development of all living things.
Phosphorus is unique from all the other cycles since it does not have a gaseous phase. Its main natural sources in biological systems are weathered rocks, decomposed organic matter, and anthropogenic inputs like fertilizers. These anthropogenic sources introduce phosphorus—an essential nutrient for aquatic organisms, and a limiting nutrient in many environments—essential for plant and algal growth.
Plants take up phosphorus through their roots and utilize it to create key compounds like ATP that fuel a plant’s metabolic activities and growth.
Phosphorus Movement in Ecosystems
Phosphorus transport within ecosystems is a complicated, multi-step process. At first, phosphorus is mined from sediments through weathering, which leads to soils and water systems. Plants take it up from the ground, and animals get phosphorus by either eating plants or eating other animals that have eaten plants.
In nature, decomposers are always hard at work recycling phosphorus by breaking down organic matter. They also refill our soils with vital nutrients, ensuring that they’re always available for reuse.
In aquatic ecosystems, phosphorus is the most important driver of water quality. It can contribute to eutrophication, in which elevated nutrient levels result in rapid algae growth that consumes oxygen and suffocates aquatic animals. Phosphorus is adsorbed by wetland soils, which plants are able to uptake, demonstrating its mobility through various environmental compartments.
Factors influencing phosphorus availability include:
-
Soil pH levels
-
Temperature
-
Microbial activity
-
Presence of mycorrhizal fungi
-
Human activities like agriculture and urbanization
Human Impact on Phosphorus Levels
However, human activities have dramatically increased the amount of phosphorus above natural levels. Agricultural runoff, loaded with fertilizers, is the major source of phosphorus pollution. This causes algae blooms in lakes and rivers, degrading water quality and harming aquatic ecosystems.
Detergents contribute to this problem as well with their chemical make-up as phosphorus is one of their main ingredients. In order to manage phosphorus sustainably, a handful of actions can be taken.
Reducing fertilizer application, encouraging use of phosphorus-free detergents and installing buffer strips in farm fields can make a difference. These strategies emphasize reducing runoff and preventing excess phosphorus from reaching water systems. In doing so, they safeguard ecosystems and maintain the equilibrium that is so critical for life.
The Sulfur Cycle
So in conclusion The sulfur cycle is fundamental to our ecosystem. It is one of the most important determinants of the health of our planet and people. This cycle illustrates the flow of sulfur as it changes from one form to another.
It comprises elemental sulfur (S0), sulfide (S2-), thiosulfate (S2O32-), tetrathionate (S4O62-), and sulfate (SO42-). Sulfur is a key component of proteins and vitamins, thus making it essential to life as we know it. The cycle’s complexity reflects its important influence on many biological processes.
For instance, sulfur is important for protein synthesis, as it is necessary in the formation of the amino acids cysteine and methionine.
Sulfur in the environment comes primarily from natural sources. Volcanic eruptions are a major natural contributor, with eruptions injecting large quantities of sulfur dioxide (SO2) into the atmosphere. Oceans are also a net emitter of sulfur compounds as a result of biological production of dimethylsulfide (DMS).
Indigenous terrestrial sources include the weathering of pre-existing geologic materials such as rocks and soil minerals that are rich in sulfur. No matter what, human activities have enhanced the natural contributions. Human activities, especially the combustion of fossil fuels and the production of sulfuric acid, emit large quantities of sulfur to the atmosphere.
In the sulfur cycle, inorganic sulfur compounds are oxidized and reduced through several different forms. This is also called mineralization, which is the process of changing organic sulfur into inorganic forms. At the same time, oxidation converts reduced sulfur to sulfate via the activities of microorganisms.
Microbial activity is especially important, as bacteria such as Thiobacillus are vitally important in the oxidation of sulfur. Microbial metabolic processes can increase sulfur oxidation rates up to three orders of magnitude over abiotic processes.
Sulfur Transformation in Nature
Mineralization and oxidation are important steps in the sulfur cycle. Mineralization transforms organic sulfur to inorganic compounds like hydrogen sulfide (H2S) or sulfate (SO42-). At the same time, oxidation turns sulfide or elemental sulfur into sulfate, often with the aid of microorganisms.
Sulfur in the atmosphere has important climate implications, as sulfates can form sulfate aerosol that reflect sunlight and cool the Earth. Windblown dust may play an important role in the sulfur cycle by transporting sulfur compounds over extremely long distances.
Bacteria are key to this effort. They help accelerate transformations and also assist in polymer biodegradation, doing so at rates of 50-60% in 18 months.
-
Volcano eruption, weathering of sulfur-rich rocks Sulfur Cycle
-
Volcanic emissions of SO2 Biological formation of dimethylsulfide in oceans Microbial oxidation and reduction of sulfur compounds Creation of sulfate aerosols in the atmosphere Incoming solar radiation and the sulfur cycle
-
Deposition of sulfur compounds to the planetary surface
Human Influence on Sulfur Cycle
Human activities, especially industrial processes, play a major role in the sulfur cycle. Combustion of fossil fuels and other industrial activities release large amounts of sulfur dioxide, which adds to total sulfur emissions.
The problem of acid rain is one of the most important environmental issues. It occurs when sulfur dioxide and nitrogen oxides react with water vapor in the atmosphere, resulting in acidic precipitation that can harm ecosystems, erode buildings, and acidify lakes and streams.
Fossil fuel combustion has greatly altered the natural sulfur cycle. As a consequence, atmospheric sulfur loading has increased, altering global sulfur budgets. Though progress has been made in reducing emissions in places such as North America and Europe, increasing emissions from Asia continue to pose formidable challenges.
To mitigate human impacts, strategies such as adopting cleaner technologies, reducing fossil fuel usage, and implementing stricter emission controls are crucial. These steps have played an important role in improving sulfur stability in the environment and reducing harmful effects such as acid rain.
The Oxygen Cycle
Our oxygen cycle is a very important biogeochemical cycle that is essential for sustaining life on Earth. The cycle also includes the movement of oxygen through the atmosphere, biosphere, and lithosphere, providing essential support for all processes of life. Oxygen is essential for cellular respiration, the way that most living organisms get their energy.
This cycle keeps oxygen circulating and available for organisms to use to produce energy, making it essential for life. Without this oxygen cycle, our world would be a very different place.
Oxygen Production and Consumption
Plants, algae, and some bacteria release oxygen as a byproduct of photosynthesis. Using energy from the sun, they turn carbon dioxide and water into glucose and oxygen. This incredible process, which takes place in the chloroplasts of plant cells, is the basis for oxygen production in nearly all of Earth’s ecosystems.
Photosynthesis not only provides oxygen but also forms the base of most food webs by creating organic matter that fuels various organisms. On the other hand, oxygen is used by organisms during respiration. In respiration, cells consume oxygen to convert glucose into usable energy, producing carbon dioxide and water as byproducts.
Both plants and animals respire, using the oxygen generated by photosynthesis and completing the cycle’s equilibrium. This balance is delicate yet important, as it is the nature of this balance that keeps oxygen abundant enough to sustain life. Ecosystems depend on a precarious balance between the production and consumption of oxygen.
Several factors, such as temperature, availability of light, and the activity of decomposers, greatly affect this equilibrium. In aquatic systems, water temperature and the quantity of organic material can lead to dramatic swings in oxygen levels. These shifts have a profound effect on the overall ecosystem.
-
Fluctuations in temperature
-
Light intensity
-
Involvement of decomposers
-
Quality of the soil
-
Organic matter content
-
Salinity of water
-
Environmental Significance
The significance of the oxygen cycle goes beyond just the individual organisms that depend on it, for it is crucial to maintaining entire ecosystems. Oxygen is the key ingredient in aerobic respiration, the process that harvests energy from organic molecules to power the activities of the planet’s billions of other organisms.
By allowing species to occupy unique niches and habitats, this cycle promotes biodiversity, which is essential for the health of our planet. When we disrupt the oxygen cycle, the ecological consequences can be profound. For instance, deforestation and pollution could negatively impact oxygen production, resulting in less oxygen available for respiration.
These types of disruptions can lead to biodiversity loss, as many species cannot endure with insufficient oxygen. Reducing oxygen levels puts both animal and plant ecosystems on the brink by altering the makeup of species. This drastic change can result in the overgrowth of anaerobic organisms, throwing off the entire ecosystem.
Major implications are tied to changing oxygen dynamics. In order to maintain the oxygen cycle, we need to protect and restore the natural environments that produce it.
The Hydrological Cycle
The hydrological cycle, or water cycle, is the never-ending process of circulating water within Earth and the atmosphere. It is this dynamic process that prevents water from ever sitting still. It’s the driving force behind Earth’s ecosystems, nurturing healthy, vibrant landscapes that sustain all life on our planet.
Water cycles through solid, liquid, and gaseous states, traveling between reservoirs including our oceans, rivers, and atmosphere. This cycle is a critical part of moving water around our planet, making it available for life to flourish in a variety of shapes and forms. The cycle’s significance goes far beyond supporting life — it helps shape our weather, climate, and even our geologic processes.
The sun provides the power for this entire cycle. It also gives us the energy that drives our hydrological cycle — it’s what pulls water out of dried up surfaces through evaporation. This complex process, shaped by natural forces and human activities, ultimately dictates where freshwater—an increasingly scarce resource essential for drinking, agriculture, and industry—is available.
Water Movement and Distribution
Evaporation and precipitation are two major processes in the hydrological cycle. Water vapor evaporates from oceans, lakes and rivers, rising high into the atmosphere, where it cools and condenses to form clouds. Eventually, this water returns to Earth as rain or snow, refilling rivers and lakes, and feeding the land.
Every year, precipitation brings roughly 107,000 km³ of liquid water onto Earth’s landmasses, and snow accounts for another 1,000 km³. When rainwater hits the ground, instead of getting absorbed into the soil, it runs off. This green infrastructure function helps to recharge aquifers and groundwater reserves, vital for supporting natural ecosystems and human uses.
Groundwater can remain tucked away below the surface of the Earth for more than 10,000 years. It continues to function as an essential saline reservoir, nurturing diverse ecosystems, including those in the most arid areas. That doesn’t mean it’s evenly distributed — the planet’s water distribution varies dramatically. Only 1.7% of Earth’s water is sequestered in ice caps, glaciers, and permanent snowpacks.
Factors influencing water movement include:
-
Temperature and solar energy levels
-
Atmospheric pressure variations
-
Geographical features like mountains and plains
-
Human activities like deforestation and urbanization
Impact on Climate Systems
The hydrological cycle plays an important role in shaping weather and climate patterns. Water vapor, the most important greenhouse gas, influences Earth’s dynamic equilibrium. Our planet’s temperature is primarily regulated by greenhouse gases, including water vapor.
Climate change is also affecting the hydrological cycle, changing weather patterns and resulting in increased risk of extreme events like droughts or floods. We know that climate change is already making the hydrological cycle more extreme. Warmer temperatures increase the rate of evaporation and change where precipitation falls.
Adding to these changes is the unprecedented level of atmospheric carbon, not seen in at least 420,000 years. To avoid long-term water scarcity, upstream strategies like restoring wetlands, increasing efficiency of water use, and protecting aquifers are key.
We also need to address the human activities that damage the cycle. Each year we still emit 24 million tons of nitrogen oxides. This pollution is a critical piece of our mitigation puzzle.
Sedimentary Cycles
Image credit: britannica
Sedimentary cycles are one of the cornerstones of the geological processes that shape the Earth. These cycles are characterized by the movement and transformation of material through erosion, transport, and deposition, ultimately leading to the creation of sedimentary rocks. Sedimentary cycles operate on significantly longer timescales than gaseous cycles.
Where gaseous cycles have carbon and nitrogen circulating through the atmosphere, sedimentary cycles are primarily concerned with the solid materials of the Earth. They are special because they are the Earth's surface. Moreover, they deliver important nutrients to ecosystems.
Sediments are incredibly important to nutrient cycling. They are giants in the global cycle, sequestering key elements such as phosphorus and sulfur. Via weathering and erosion, they slowly return these elements back to the environment.
This gradual release not only ensures a continuous flow of nutrients to ecosystems but also stimulates plant growth, enabling the development of healthy and fertile soil. Sedimentary rocks are formed over the course of millions of years. They function as a huge battery, safely sequestering vital nutrients until the biosphere can use them.
Role in Earth’s Crust Formation
Sedimentary cycles are fundamental to creating continental crust on Earth. For one, they help break down rocks through the process of weathering. Next, wind, water, or ice carry the particles elsewhere.
Once transported, these materials become deposited, settling out and depositing commonly in layers resulting in deposition. Through this process, called lithification, the layers are compacted and cemented into sedimentary rocks. This tectonics teaches us about the process that forms the very structure of the Earth’s crust.
It also protects a wonderful and irreplaceable record of the earth’s geological history. Sedimentary rocks contain important information about ancient climates, ecosystems, and tectonics. Scientists who study the history of our planet find these rocks to be priceless.
-
Weathering: Breakdown of rocks into smaller particles
-
Transport: Movement of sediments by wind, water, or ice
-
Deposition: Accumulation of sediments in new locations
-
Lithification: Compaction and cementation into solid rock
Interaction with Other Cycles
Sedimentary cycles are tightly coupled with other biogeochemical cycles, both affecting and being affected by them. Sediments are important regulators of nutrient availability for ecosystems. They serve as both source and sink of nutrients.
They can hoard nutrients in excess times and release them during lean times, which can help keep ecosystems in dynamic equilibrium. However, human activities such as mining and land development often destroy, damage, or otherwise disconnect these natural cycles.
This disruption contributes to erosion and the decay of important sedimentary habitats. To safeguard these critical ecosystems and the services they provide, solutions like holistic land management and smart conservation investments are key.
These strategies bring us closer to restoring the natural balance of sedimentary cycles and bolstering the health of our ecosystems.
Human-Induced Changes in Cycles
Human activities have profoundly impacted natural biogeochemical cycles, resulting in widespread ecological consequences. This human-induced change is mostly due to industrial, agricultural, and urban development. By burning fossil fuels, humanity is pumping massive amounts of carbon into the atmosphere.
In fact, as recently as 2018, an estimated 36.6 gigatons were added. That sudden influx of carbon – about 400 billion tons of it – totally disrupted the carbon cycle, which had been in balance for millennia. Then we ask why every year we still emit close to 24 million tons of nitrogen oxides into the air.
This pollution fuels climate change and causes problems like acid rain. Human-induced nitrogen fixation, which contributes another 5-8% to the total nitrogen fixed, further upsets natural balances. At the same time, we harvest about 25% of the terrestrial plant biomass produced each year, disrupting the nutrient cycles on land.
Impacts on Global Climate
Human-induced changes in biogeochemical cycles are already making deep and lasting impressions on the global climate. This surplus carbon from burning fossil fuels is filling the atmosphere with greenhouse gases, intensifying the impacts of climate change.
These disruptions lead to feedback loops, in which warming temperatures are changing these cycles even more, exacerbating the impacts from climate change. Increasing the amount of carbon in the atmosphere increases global temperatures.
This increase, in turn, influences ecosystems’ capacity to uptake carbon and sequester it in long-term carbon stores. Key impacts of human-induced changes on climate include:
-
Increased global temperatures due to higher carbon levels.
-
Altered precipitation patterns resulting from changes in atmospheric composition.
-
Increasingly frequent and severe weather events attributed to climate change.
-
Ocean acidification from increased carbon dioxide absorption.
-
Continuing loss of biodiversity as ecosystems can’t keep up with the pace and scale of human-caused changes.
Strategies for Mitigation
To reduce anthropogenic pressures on biogeochemical cycles, we can take a variety of steps. Focusing on environmentally sustainable agricultural and industrial production methods is needed.
We can lessen the carbon burden on the atmosphere by cutting nitrogen oxide emissions. Further, reducing our dependence on fossil fuels will ensure that we can do so. Conservation efforts are key to cycle restoration, protecting ecosystems and restoring habitats protect the natural world and world’s biodiversity.
Policies and practices that promote cycle restoration include:
-
Implementing renewable energy sources to decrease carbon emissions.
-
Encouraging organic farming to reduce chemical inputs in agriculture.
-
Enhancing reforestation efforts to increase carbon sinks.
-
Regulating industrial emissions to minimize atmospheric pollutants.
-
Supporting research and development focused on sustainable technologies.
Conclusion
These biogeochemical cycles are incredibly important in keeping all of our planet’s ecosystems in balance. Each one, carbon, nitrogen, oxygen, sulfur, cycles, helps to make our planet as vibrant as it is today. These cycles are interconnected and essential to all forms of life on Earth, cycling nutrients and energy. Human activities can throw this careful balance out of whack. Our decisions also interfere with these natural processes, with consequences for climate, air quality, and biodiversity.
Let’s work to advance sustainable practices and smart innovations that safeguard these cycles. By recognizing our role in these natural cycles, we can all work together to create a healthier planet. Learn more about these important cycles and find out how you can help protect them in your community. Together we can create a sustainable future, so take action today. Together, we can make sure the Earth is a wonderful place for our children’s children to enjoy.
Frequently Asked Questions
What Are Biogeochemical Cycles?
Biogeochemical cycles are natural processes that recycle essential nutrients, such as carbon, nitrogen, and phosphorus, in different chemical forms. For example, they govern the movement of essential building blocks to life such as carbon, nitrogen and phosphorus through ecosystems. These complex cycles are essential for the maintenance of life on our planet.
How Does the Carbon Cycle Work?
The carbon cycle describes the movement of carbon through the atmosphere, oceans, soil, and biosphere. Plants take up carbon dioxide to produce their energy through photosynthesis, while animals put carbon back into the atmosphere through respiration. The combustion of fossil fuels, in contrast, breaks this balance, adding more carbon to the atmosphere.
Why Is the Nitrogen Cycle Important?
The nitrogen cycle is the process by which atmospheric nitrogen is converted into forms that plants and animals can use. It’s also critical for synthesizing proteins and nucleic acids. However, human activities such as agriculture can disrupt this cycle, resulting in significant environmental impacts.
What Role Does the Phosphorus Cycle Play in Ecosystems?
The cyclical model of the phosphorus cycle describes the movement of phosphorus through soil, water, and living organisms. It’s also essential for DNA synthesis and ATP production. Unlike the other cycles, it doesn’t include the atmosphere which makes it much slower and more localized.
How Does Human Activity Affect These Cycles?
However, anthropogenic activities, including industrialization and commercial agriculture, have drastically changed biogeochemical cycles. They worsen climate change by increasing greenhouse gas emissions and aggravate nutrient runoff, contributing to climate change and nutrient pollution. These impacts are complex but understanding them is essential to creating a more sustainable world.
What Is the Hydrological Cycle?
The hydrological cycle , or water cycle, refers to the movement of water on, above and below the Earth’s surface, in an endless cycle. It encompasses the water cycle—processes such as evaporation, condensation, and precipitation, which are integral to circulating fresh water.
What Are Sedimentary Cycles?
Sedimentary cycles are the transport of nutrients through sedimentary rock layers. Other elements such as phosphorus and sulfur are also cycled in this manner. These cycles are much more gradual than gaseous cycles, severely affecting long-term soil fertility.
What's Your Reaction?














